
Based on model-building studies, Spaepen 19 has calculated that the configurational entropy due to lattice disorder in the amorphous phases is approximately 0.2 k. The molar entropy offsets, however, can only be tentatively estimated. In particular, the molar enthalpies of the amorphous phases relative to the molar enthalpies of the crystal phases are fixed by the latent heats of crystallization, which have been measured 18 to be 0.139 eV/atom ≈ at 950 K for Si and to be 0.119 eV/atom at ≈ 720 K for Ge. Since both 〈Si〉 c and 〈Ge〉 c are the equilibrium phases of Si or Ge at standard temperature and pressure, we set their enthalpies to zero at 298 K.įor the amorphous solid phases, we can estimate fairly well the molar enthalpy offsets relative to the molar enthalpies of the crystalline phases. The enthalpy scale, however, is not fixed by an equivalent law, so, as is customary, we arbitrarily fix the enthalpy of the equilibrium phases of Si or Ge at standard temperature and pressure (298 K and 760 Torr) to be zero. Therefore, we can use as the entropy offset the entropy at 0 K, namely zero. A fit of the experimental data to equation (8.31)įor the crystalline phases, the third law of thermodynamics states that the entropy at absolute zero of a perfect crystalline substance is zero. A solid phase transition occurs in c-C 6H 12 at T = 186.12 K, resulting in a second invariant point in the phase diagram at this temperature and x 2 = 0.6115, where liquid and the two solid forms of c-C 6H 12 are in equilibrium. The (solid + liquid) phase diagram for ( x 1n-C 6H 14 + x 2c-C 6H 12) has a eutectic at T = 170.59 K and x 2 = 0.3317.

Plot these results on the diagram constructed in part (a) to compare the actual freezing points with the ideal solution prediction. Assume this Δ fus H m is constant with temperature and calculate the ideal freezing point at every 0.1 mole fraction unit.
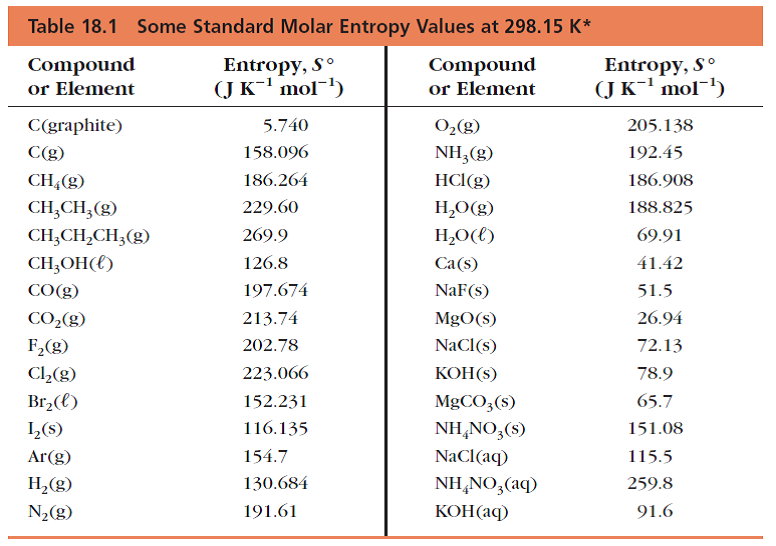
Use the ( T, x 2) data near x 2 = 0 and x 2 = 1 to calculate Δ fus H m for both components. Ĭonstruct the binary (solid + liquid) phase diagram and label all the regions. For a more detailed description of the technique, the reader is referred to more comprehensive and dedicated references. To relate the measured temperature effect to the value of the enthalpy of the reaction of dissolution or precipitation, electrical calibrations are moreover performed so as to determine the energy equivalent of the system. The reaction taking place can be one of dissolution or precipitation depending on the particular situation, although precipitation can lead to poorly defined crystalline states and therefore should be considered with caution. (1.39) Np ( cr ) + O 2 ( g ) = NpO 2 ( cr ) Δ c o m b H ° m ( Np, cr, 298.15 K ) = Δ f H ° m ( NpO 2, cr, 298.15 K )Ī solution calorimetry experiment consists of dissolving a sample that can be either solid or liquid into an solution, and measuring the temperature rise (for an exothermic reaction where heat is released) or decrease (for an endothermic reaction where heat is absorbed) following contact between the sample and solution.


 0 kommentar(er)
0 kommentar(er)
